A concerning signal: CDC-linked study finds higher hypertension risk in vaccinated pregnancies
02/14/2026 / By Willow Tohi
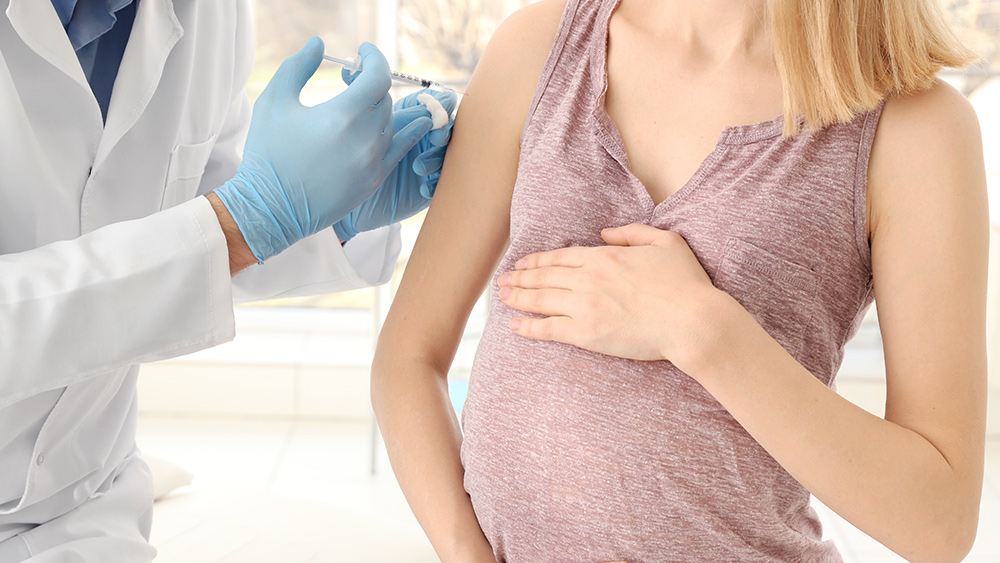
- A new CDC-affiliated study finds a 24% higher reported risk of hypertensive disorders in vaccinated first-time pregnancies.
- The peer-reviewed research analyzed over 16,000 pregnancies from CDC registries.
- Authors stress the findings are associative, not causal, but call for updated risk-benefit analyses.
- The elevated risk was similar in magnitude to that associated with contracting COVID-19 during pregnancy.
- The CDC’s current formal guidance on COVID-19 vaccination during pregnancy is listed as “not applicable.”
In a development that challenges longstanding official assurances, a new study conducted by researchers affiliated with the Centers for Disease Control and Prevention (CDC) has identified a statistically significant increase in serious pregnancy-related hypertensive disorders among women who received a COVID-19 vaccine. Published in the peer-reviewed journal Vaccine in February 2026, the analysis of more than 16,000 first-time pregnancies found vaccinated women had a 24% higher reported risk of these potentially life-threatening conditions compared to their unvaccinated counterparts. The findings, which the authors caution do not prove causation, nonetheless introduce a critical new dimension to the ongoing debate over the risks and benefits of COVID-19 vaccination during pregnancy, a policy aggressively promoted by U.S. health authorities since 2021.
The study’s key findings: A statistically significant association
The registry-based study compared outcomes from two CDC systems: the COVID-19 Vaccine Pregnancy Registry (for vaccinated women) and the Pregnancy Risk Assessment Monitoring System (for unvaccinated women). To ensure a fair comparison, researchers meticulously matched participants one-to-one based on age, race, ethnicity and gestational age at delivery, focusing solely on first-time mothers with single pregnancies and excluding those with pre-existing chronic hypertension.
The results were clear. Among the matched pairs, 15% of vaccinated participants reported a hypertensive disorder of pregnancy (HDP), such as gestational hypertension or preeclampsia, compared to 12% of unvaccinated women. After adjusting for additional factors like body mass index and diabetes, the elevated risk persisted. Notably, the increased risk was observed regardless of which COVID-19 vaccine manufacturer was used or during which trimester the vaccination occurred. Perhaps most strikingly, the study found the magnitude of risk associated with vaccination was similar to the risk associated with contracting COVID-19 illness during pregnancy, which carried a 28% higher HDP risk.
From emergency use to urgent recommendation
This new data stands in stark contrast to the public health messaging that defined the pandemic for pregnant women. In April 2021, then-CDC Director Dr. Rochelle Walensky began urgently recommending COVID-19 vaccination for all pregnant people, stating the shots were safe despite acknowledging “limited data” on pregnancy safety at the time. This recommendation was based largely on a retrospective study published in the New England Journal of Medicine, which critics argued was skewed because most participants were vaccinated in their third trimester, obscuring potential first-trimester risks like miscarriage.
- Health authorities promoted vaccination as a safe, vital protection for pregnant individuals, who were classified as high-risk for severe COVID-19.
- Major medical organizations, including the American College of Obstetricians and Gynecologists (ACOG), continue to recommend the vaccines, with ACOG’s website stating they are “completely safe” for use in pregnancy.
- The new study directly questions the foundation of those blanket safety assurances by identifying a significant association with serious maternal health complications.
Plausible biological mechanisms: Science suggests a pathway
The study authors did not merely report a statistical link; they explored biologically plausible mechanisms that could explain the findings. A primary focus was the role of the SARS-CoV-2 spike protein and its interaction with angiotensin-converting enzyme 2 (ACE2) receptors, which are abundant on placental cells and play a key role in blood pressure regulation. Since mRNA vaccines instruct the body’s cells to produce the spike protein, researchers have noted this could theoretically lead to temporary blood pressure increases. The authors also pointed to vaccine-induced immune activation and inflammation as a potential contributor, as inflammatory responses can disrupt placental blood flow early in pregnancy, a known pathway to hypertensive disorders.
Limitations and the call for renewed scrutiny
The researchers were careful to outline the study’s limitations, preventing a rush to causal judgment. Key limitations include:
- Reliance on self-reported diagnoses, though validation checks showed strong alignment with medical records.
- Differences between the vaccinated and unvaccinated cohorts; nearly half of the vaccinated group were healthcare workers, who experienced unique pandemic stresses and may have had different health monitoring practices.
- The two groups were drawn from different surveillance systems and slightly different time periods.
Consequently, the authors emphasized their results “cannot be considered causal.” However, they concluded that “updated assessments of HDP risks after illness and vaccination would be useful.” This echoes long-standing calls from independent scientists and advocates for more rigorous, prospective safety studies before mandating medical interventions for pregnant populations.
An unsettled legacy and a path forward
The publication of this CDC-linked study marks a pivotal moment in the narrative of COVID-19 vaccine safety during pregnancy. It provides peer-reviewed, data-driven evidence that contradicts earlier absolute safety claims and underscores the complexity of risk-benefit analyses in real-time public health. While the CDC’s formal guidance on the matter now appears in a state of limbo—listed as “no guidance/not applicable” on its pregnancy webpage—the study demands a sober reevaluation. For the scientific community and health authorities, it reinforces the imperative of robust, transparent safety surveillance. For pregnant individuals and their families, it highlights the necessity of informed consent based on evolving, and sometimes unsettling, data. The quest for clarity on the full impact of pandemic policies continues, with the health of mothers and children remaining the ultimate measure of their success or failure.
Sources for this article include:
Submit a correction >>
Tagged Under:
Big Pharma, biological weapon, CDC, Censored Science, COVID, medical violence, pandemic, pharmaceutical fraud, pregnant women, research, spike protein, vaccine wars, vaccines, women's health
This article may contain statements that reflect the opinion of the author
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 REAL SCIENCE NEWS